how to reduce ketone to alcohol Lialh4 reduction of ketone and aldehyde
Ketones and aldehydes are important functional groups in organic chemistry, and their reduction is a crucial step in various synthetic pathways. In this post, we will explore the mechanism of ketone and aldehyde reduction by NaBH4 and LiAlH4, two commonly used reducing agents.
The Mechanism of Ketone Reduction by NaBH4
 NaBH4, also known as sodium borohydride, is widely used as a reducing agent in organic synthesis. When NaBH4 reacts with a ketone, it forms a complex intermediate known as an alkoxyborohydride. This intermediate is then protonated, resulting in the formation of an alcohol and borate ester.
NaBH4, also known as sodium borohydride, is widely used as a reducing agent in organic synthesis. When NaBH4 reacts with a ketone, it forms a complex intermediate known as an alkoxyborohydride. This intermediate is then protonated, resulting in the formation of an alcohol and borate ester.
The reduction of ketones by NaBH4 is stereoselective, meaning that it usually leads to the formation of a single stereoisomer. The stereochemistry of the product is determined by the steric interactions between the bulky borohydride ion and the substituents on the ketone.
It is important to note that NaBH4 is selective towards reducing ketones and aldehydes but does not react with other functional groups commonly found in organic compounds, such as double bonds or carboxylic acids.
The Mechanism of Ketone Reduction by LiAlH4
 LiAlH4, also known as lithium aluminum hydride, is a very powerful reducing agent. Unlike NaBH4, LiAlH4 can reduce various functional groups, including ketones, aldehydes, esters, carboxylic acids, and even certain types of halides.
LiAlH4, also known as lithium aluminum hydride, is a very powerful reducing agent. Unlike NaBH4, LiAlH4 can reduce various functional groups, including ketones, aldehydes, esters, carboxylic acids, and even certain types of halides.
Similar to NaBH4, the reduction of a ketone by LiAlH4 proceeds through the formation of an intermediate. In this case, the intermediate is a complex between the ketone and the hydride ion. This complex is then protonated, leading to the formation of an alcohol and aluminum-based compound.
The reduction of ketones by LiAlH4 is not only stereoselective but also regioselective. This means that the reaction usually occurs at one specific carbon atom within the ketone molecule. The selectivity can be influenced by factors such as steric hindrance and electronic effects.
In summary, the reduction of ketones and aldehydes by NaBH4 and LiAlH4 involves the formation of intermediate complexes, which are subsequently protonated to yield alcohols. NaBH4 is selective towards ketones and aldehydes, while LiAlH4 can reduce a wider range of functional groups. Understanding these reduction mechanisms is essential for designing efficient synthetic routes in organic chemistry.
If you are searching about Addition of NaBH4 to ketones to give secondary alcohols – Master you’ve came to the right page. We have 5 Images about Addition of NaBH4 to ketones to give secondary alcohols – Master like Addition of NaBH4 to ketones to give secondary alcohols – Master, Selective reduction of aldehydes and ketones to alcohols with ammonia and also LiAlH4 Reduction of Ketone and Aldehyde - the Mechanism | Chemistry. Read more:
Addition Of NaBH4 To Ketones To Give Secondary Alcohols – Master
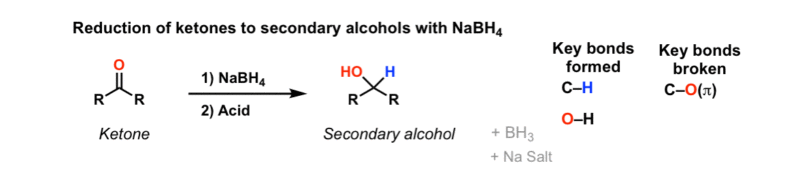 www.masterorganicchemistry.comketones nabh4 alcohols reduction addition
www.masterorganicchemistry.comketones nabh4 alcohols reduction addition
Selective Reduction Of Aldehydes And Ketones To Alcohols With Ammonia
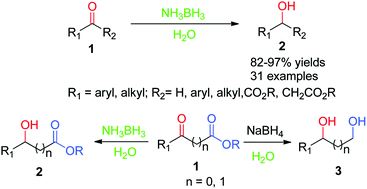
LiAlH4 Reduction Of Ketone And Aldehyde - The Mechanism | Chemistry
 www.pinterest.co.uklialh4 reduction carbonyl nabh4 ketone aldehyde alcohols borohydride ketones carboxylic hydride addition citric ester sodium chemistrysteps reduktion oxidation mechanismus hydrogen
www.pinterest.co.uklialh4 reduction carbonyl nabh4 ketone aldehyde alcohols borohydride ketones carboxylic hydride addition citric ester sodium chemistrysteps reduktion oxidation mechanismus hydrogen
LiAlH4 Reduction, Ketone To Alcohol Mechanism | Organic Chemistry - YouTube
 www.youtube.comketone reduction alcohol mechanism lialh4 chemistry
www.youtube.comketone reduction alcohol mechanism lialh4 chemistry
The Mechanism Of Ketone And Aldehyde Reduction By NaBH4 | Organic
 www.pinterest.clreduction mechanism nabh4 chemistry ketone organic aldehyde sodium carbonyl borohydride lialh4 ketones alcohol carboxylic ester aldehydes group nabh reactions between
www.pinterest.clreduction mechanism nabh4 chemistry ketone organic aldehyde sodium carbonyl borohydride lialh4 ketones alcohol carboxylic ester aldehydes group nabh reactions between
Lialh4 reduction carbonyl nabh4 ketone aldehyde alcohols borohydride ketones carboxylic hydride addition citric ester sodium chemistrysteps reduktion oxidation mechanismus hydrogen. Borane selective aldehydes ketones ammonia alcohols. Selective reduction of aldehydes and ketones to alcohols with ammonia